Hard Carbon (Grade I & II)
HC
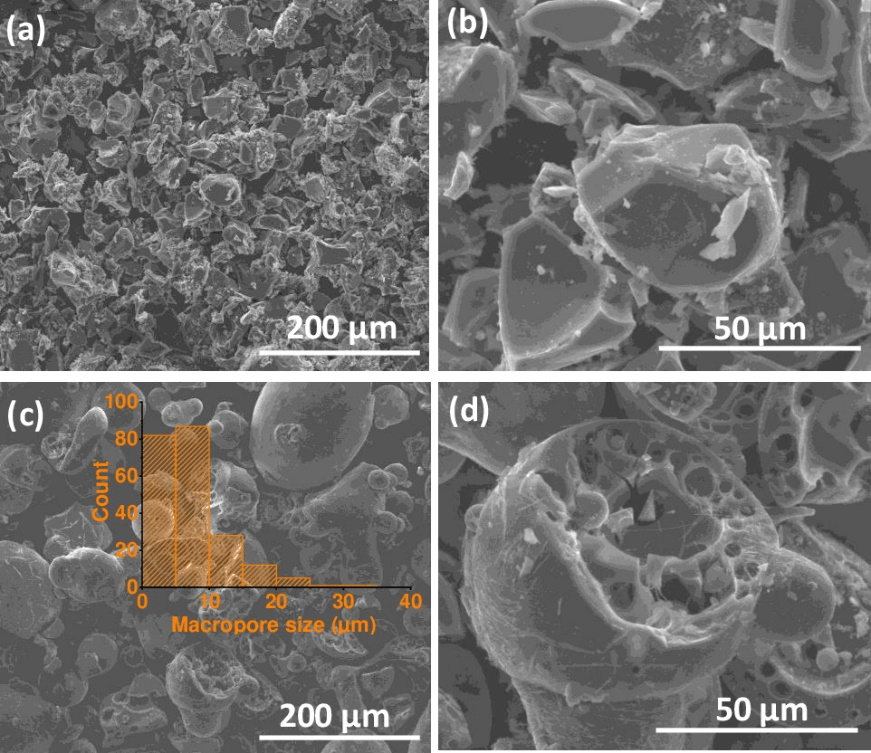
Hard Carbon is a crucial anode material for sodium-ion batteries. Known for its excellent electrochemical properties, it offers high capacity and long cycle life. Hard Carbon has a disordered structure that provides ample sites for sodium ion storage and easy diffusion pathways.
-
Appearance: Black powder
-
Particle Size Distribution:
-
Grade I: D50: 3-5 µm
-
Grade II: D50: 5-8 µm
-
-
Specific Surface Area: 5-15 m²/g
-
Purity: ≥ 99.9%
-
Conductivity: ≥ 10 S/cm
Hard carbon is a solid form of carbon that cannot be converted to graphite by heat-treatment, even at temperatures as high as 3000 °C. It is also known as char, or non-graphitizing carbon. More colloquially it can be described as charcoal.
Hard carbon is produced by heating carbonaceous precursors to approximately 1000 °C in the absence of oxygen. Among the precursors for hard carbon are polyvinylidene chloride (PVDC), lignin and sucrose. Other precursors, such as polyvinyl chloride (PVC) and petroleum coke, produce soft carbon, or graphitizing carbon. Soft carbon can be readily converted to graphite by heating to 3000 °C.
The physical properties of the two classes of carbons are quite different. Hard carbon is a low density material, with extremely high microporosity, while soft carbon has little microporosity. Hard carbon is extensively used as anode materials in lithium-ion batteries and sodium-ion batteries.
Specification
Grade: Battery
Type: Anode & Cathode
Size: 100 g (black powder)
Charge-Discharge Data:
-
Initial Coulombic Efficiency: ≥ 75%
-
Specific Capacity:
-
Initial Discharge Capacity: 300-350 mAh/g
-
Capacity Retention: ≥ 90% after 100 cycles
-
-
Charge/Discharge Rate Capability:
-
At 0.1C: 320-340 mAh/g
-
At 1C: 280-300 mAh/g
-
At 5C: 200-220 mAh/g
-
-
Cycling Stability: Less than 10% capacity fade over 100 cycles at 0.5C
-
Voltage Window: 0.01V to 2.0V (vs. Na/Na⁺)
Additional Data:
-
XRD Patterns: Show a broad peak indicating amorphous carbon.
-
SEM: Displays a consistent and uniform particle morphology.
-
EIS: Indicates low impedance and efficient sodium ion transport.